This post is a repost of a North Carolina State University press release: In Ironic Twist, CRISPR System Used to Befuddle Bacteria
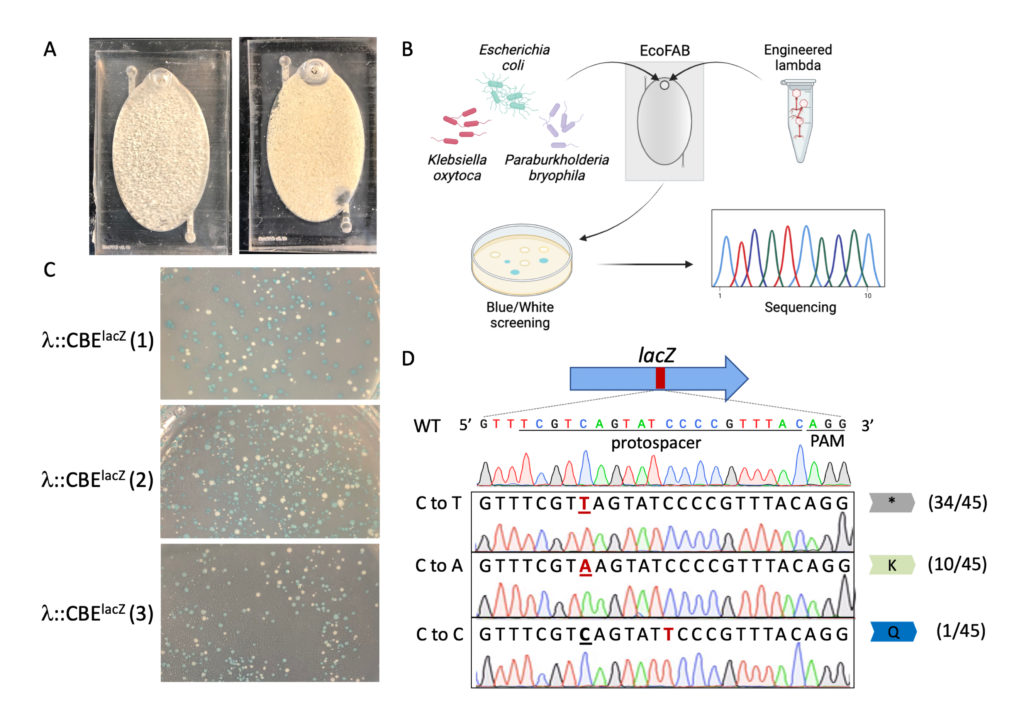
Base editing within a community context. (A) An EcoFAB device filled with quartz sand (Left) and an EcoFAB device loaded with LB and synthetic soil community (Right). (B) EcoFAB community editing strategy overview. (C) Blue or white E. coli colonies resulting from synthetic community editing after infection with λ::CBE lacZ . (D) Editing was confirmed by Sanger sequencing.
Call it a CRISPR conundrum.
Bacteria use CRISPR-Cas systems as adaptive immune systems to withstand attacks from enemies like viruses. These systems have been adapted by scientists to remove or cut and replace specific genetic code sequences in a variety of organisms.
But in a new study, North Carolina State University researchers show that viruses engineered with a CRISPR-Cas system can thwart bacterial defenses and make selective changes to a targeted bacterium – even when other bacteria are in close proximity.
“Viruses are very good at delivering payloads. Here, we use a bacterial virus, a bacteriophage, to deliver CRISPR to bacteria, which is ironic because bacteria normally use CRISPR to kill viruses,” said Rodolphe Barrangou, the Todd R. Klaenhammer Distinguished Professor of Food, Bioprocessing and Nutrition Sciences at NC State and corresponding author of a paper describing the research published today in Proceedings of the National Academy of Sciences. “The virus in this case targets E. coli by delivering DNA to it. It’s like using a virus as a syringe.”
The NC State researchers deployed two different engineered viruses, or phages, to deliver CRISPR-Cas payloads for targeted editing of E. coli, first in a test tube and then within a synthetic soil environment created to mimic soil – a complex environment that can harbor many types of bacteria.
Both the engineered phages, T7 and λ (lambda), successfully found and then delivered payloads to the E. coli host on the lab bench. These payloads expressed bacterial florescent genes and manipulated the bacterium’s resistance to an antibiotic.
The researchers then used λ to deliver a so-called cytosine base editor to the E. coli host. Rather than CRISPR’s sometimes harsh cleaving of DNA sequences, this base editor changed just one letter of E. coli’s DNA, showing the sensitivity and precision of the system. These changes inactivated certain bacterial genes without making other changes to E. coli. “We used a base editor here as a kind of programmable on/off switch for genes in E. coli. Using a system like this, we can make highly precise single-letter changes to the genome without the double-strand DNA breakage commonly associated with CRISPR-Cas targeting.” says Matthew Nethery, the lead author of the study.
Model grass Brachypodium distachyon growing in sterile EcoFAB 2.0 growth chambers
Finally, the researchers demonstrated on-site editing through the use of a fabricated ecosystem (EcoFAB) loaded with a synthetic soil medium of sand and quartz, along with liquid, to mimic a soil environment. The researchers also included three different types of bacteria to test if the phage could specifically locate E. coli within the system. “This technology is going to enable our team (and others) to discover the genetic basis of key bacterial interactions with plants and other microbes within highly controlled laboratory environments such as EcoFABs” says Trent Northen, a scientist at Berkeley Lab working with Barrangou. I
“In a lab, scientists can oversimplify things,” Barrangou said. “It’s preferable to model environments, so rather than soup in a test tube, we wanted to examine real-life environments.”
The researchers inserted λ into the fabricated ecosystem. It showed good efficiency in finding E. coli and making the targeted genetic changes.
“We see this as a mechanism to aid the microbiome. We can make a change to a particular bacterium and the rest of the microbiome remains unscathed,” Barrangou said. “This is a proof of concept that could be employed in any complex microbial community, which could translate into better plant health and better gastrointestinal tract health – environments of importance to food and health.
“Ultimately this study represents the next chapter of CRISPR delivery – using viruses to deliver CRISPR machinery in a complex environment.”
The researchers plan to further this work by testing the phage CRISPR technique with other soil-associated bacteria. Importantly, this illustrates how soil microbial communities can be manipulated to control the composition and function of bacteria associated with plants in fabricated ecosystems to understand how to enhance plant growth and promote plant health, which is of broad interest for sustainable agriculture. Funding was provided by m-CAFEs Microbial Community Analysis & Functional Evaluation in Soils, a Science Focus Area led by Lawrence Berkeley National Laboratory and supported by the U.S. Dept. of Energy under contract no. DE-AC02-05CH11231, with collaborative efforts including UC Berkeley and the IGI. Co-authors of the paper include former NC State Ph.D. student Matthew Nethery, former NC State post-doctoral researcher Claudio Hidalgo-Cantabrana and NC State graduate student Avery Roberts.
Nethery, M; C. Hidalgo-Cantabrana, A. Roberts R. Barrangou (2022) CRISPR-based engineering of phages for in situ bacterial base editing. Published: Nov. 7, 2022 in Proceedings of the National Academy of Sciences [DOI]:10.1073/pnas.2206744119
This post was adapted from a North Carolina State University press release